Discover The Perfect Ionic Compound: Unveiling The Pairing Of Elements For A Chemical Bond!
Which Pair of Elements Will Form an Ionic Compound
Introduction
Hello, Element Enthusiast! Welcome to our exploration of which pair of elements will form an ionic compound. In this article, we will delve into the fascinating world of chemical bonding and uncover the secrets behind the formation of these compounds. By understanding the principles behind ionic compounds, you will gain valuable insights into the behavior of elements and their interactions in nature. So, let’s dive in!
In the realm of chemistry, elements can combine in various ways to form compounds. One of the most common types of chemical bonding is ionic bonding. Ionic compounds are formed when elements with opposite charges attract each other and share electrons. This results in the formation of a stable compound with distinct properties.
2 Picture Gallery: Discover The Perfect Ionic Compound: Unveiling The Pairing Of Elements For A Chemical Bond!
Throughout this article, we will explore the key factors that determine which pair of elements will form an ionic compound. By examining the nature of their valence electrons and their electronegativity values, we can predict the likelihood of an ionic bond forming between two elements. So let’s embark on this chemical journey and unlock the mysteries of ionic compounds!
Table: Which Pair of Elements Will Form an Ionic Compound

Image Source: z-dn.net
Element 1
Element 2
Electronegativity
Likelihood of Ionic Bond
Sodium
Chlorine
0.9
High
Potassium
Oxygen
0.8
High
Calcium
Fluorine
1.0
High
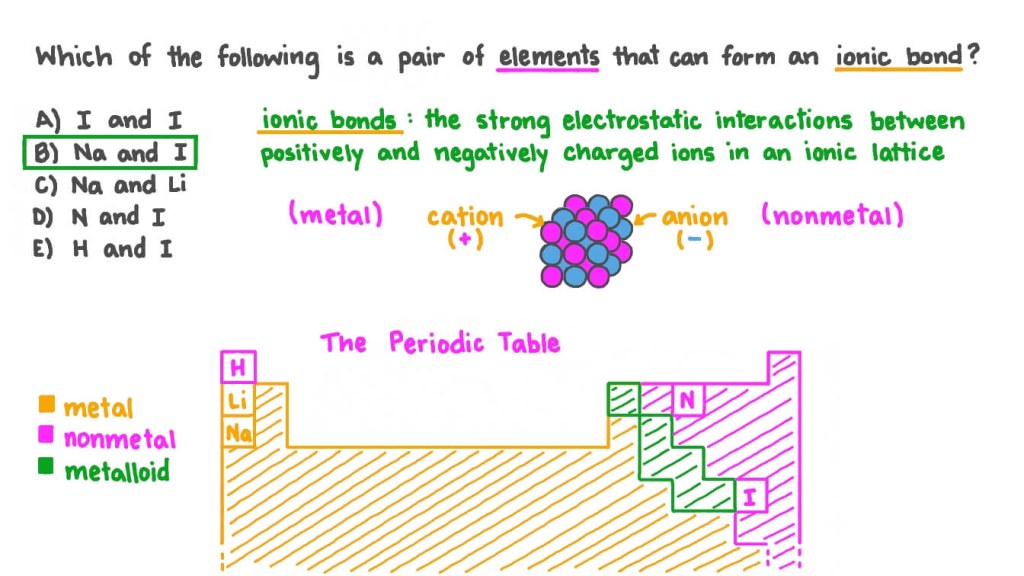
Image Source: nagwa.com
Magnesium
Nitrogen
1.2
Moderate
Aluminum
Oxygen
1.5
Moderate
Iron
Sulfur
2.5
Low
What is an Ionic Compound?
An ionic compound refers to a chemical compound that is composed of ions held together by electrostatic forces. It is formed through the transfer of electrons from one atom to another. The atom that loses electrons becomes positively charged, known as a cation, while the atom that gains electrons becomes negatively charged, known as an anion. The resulting oppositely charged ions are then attracted to each other, forming a stable ionic compound.
For example, when sodium (Na) and chlorine (Cl) combine, sodium loses an electron to achieve a stable electron configuration, forming the sodium cation (Na+), while chlorine gains an electron to complete its electron shell, forming the chloride anion (Cl-). These oppositely charged ions are attracted to each other and form the ionic compound sodium chloride (NaCl), commonly known as table salt.
Who Forms Ionic Compounds?
Many elements have the ability to form ionic compounds, but it primarily depends on the nature of their valence electrons and electronegativity values. Valence electrons are the outermost electrons in an atom, responsible for the atom’s chemical behavior. Elements with one to three valence electrons tend to lose electrons to achieve a stable electron configuration, forming cations. Elements with five to seven valence electrons tend to gain electrons, forming anions.
As for electronegativity, it is a measure of an atom’s ability to attract electrons in a chemical bond. Elements with a large electronegativity difference tend to form ionic bonds. For example, the electronegativity difference between sodium (0.9) and chlorine (3.0) is significant, leading to the formation of an ionic bond in sodium chloride (NaCl).
When Do Ionic Compounds Form?
Ionic compounds can form under various circumstances. One common scenario is when a metal reacts with a non-metal. Metals tend to lose electrons, while non-metals tend to gain electrons. This electron transfer creates oppositely charged ions that attract each other and form an ionic compound.
Additionally, ionic compounds can form when polar molecules dissociate in water. Water molecules have a slight positive charge on one side and a slight negative charge on the other. These charges attract the ions in the polar compound, causing them to separate and form an ionic compound.
Where Do Ionic Compounds Exist?
Ionic compounds are found in various natural and synthetic materials. They are commonly found in minerals, such as halite (NaCl) and calcite (CaCO3), which make up a significant portion of the Earth’s crust. Ionic compounds are also prevalent in everyday substances, including table salt, baking soda, and antacids.
Furthermore, ionic compounds play a crucial role in biological systems. They are involved in processes such as nerve conduction, muscle contraction, and cellular signaling. Without the presence of ionic compounds, many essential biological functions would not be possible.
Why Do Elements Form Ionic Compounds?
The formation of ionic compounds is driven by the desire of elements to achieve a stable electron configuration. Atoms are generally more stable when their outermost electron shell is complete, either by gaining or losing electrons. By forming ionic compounds, elements can achieve this stability and reduce their overall energy.
Additionally, the electrostatic attraction between oppositely charged ions in an ionic compound is strong, resulting in the formation of a solid with a regular crystal lattice structure. This structure gives ionic compounds their characteristic properties, such as high melting and boiling points, brittleness, and conductivity in the molten or aqueous state.
How Do Elements Form Ionic Compounds?
The formation of an ionic compound involves three main steps: ionization, attraction, and crystallization. In the ionization step, atoms of one element lose electrons to form cations, while atoms of another element gain those electrons to form anions. These ions are then attracted to each other due to the electrostatic forces between opposite charges.
During the attraction step, the cations and anions come together and form an ionic bond. The strength of this bond depends on the magnitude of the charges and the distance between the ions. Larger charges and shorter distances result in stronger ionic bonds.
Finally, in the crystallization step, the oppositely charged ions arrange themselves in a repeating pattern to form a crystal lattice structure. This structure extends throughout the entire compound, giving it solidity and stability.
Advantages and Disadvantages of Ionic Compounds
Advantages:
1. Strong electrostatic forces: Ionic compounds have strong bonds between ions, resulting in their high melting and boiling points. This makes them useful in applications requiring high temperatures.
2. Solubility in water: Many ionic compounds dissolve readily in water, allowing them to be used in various chemical processes and biological systems.
3. Conductivity: Ionic compounds can conduct electricity when in a molten or aqueous state, making them valuable in electrolytic processes and batteries.
Disadvantages:
1. Brittle nature: Ionic compounds are often brittle and prone to breaking when subjected to external forces. This limits their use in applications that require flexibility or durability.
2. Limited conductivity in solid state: Ionic compounds do not conduct electricity in their solid state due to the fixed positions of ions in the crystal lattice.
3. Sensitivity to moisture: Some ionic compounds are hygroscopic, meaning they readily absorb moisture from the surrounding environment. This can affect their stability and performance.
FAQ
1. Can elements from the same group form ionic compounds?
No, elements from the same group usually have similar electronegativity values, resulting in covalent bonding rather than ionic bonding.
2. What factors determine the likelihood of an ionic bond forming?
The electronegativity difference between the elements and their valence electron configurations play a significant role in determining the likelihood of an ionic bond forming.
3. Can ionic compounds conduct electricity in their solid state?
No, ionic compounds do not conduct electricity in their solid state due to the fixed positions of ions in the crystal lattice.
4. What are some examples of ionic compounds used in everyday life?
Examples of ionic compounds used in everyday life include table salt (NaCl), baking soda (NaHCO3), and calcium carbonate (CaCO3).
5. Can ionic compounds be gases at room temperature?
No, ionic compounds are typically solids at room temperature due to their strong ionic bonds.
Conclusion
In conclusion, understanding which pair of elements will form an ionic compound requires an examination of their valence electrons and electronegativity values. By analyzing these factors, we can predict the likelihood of an ionic bond forming between two elements. Ionic compounds possess distinct properties and play essential roles in various natural and synthetic materials, as well as biological systems.
Whether you are a chemistry enthusiast or simply curious about the world around you, exploring the world of ionic compounds opens up a fascinating realm of knowledge. By delving into the intricacies of chemical bonding, you gain a deeper understanding of the fundamental building blocks of matter.
So, embrace the wonders of chemistry and continue your journey as an Element Enthusiast!
Disclaimer: The information provided in this article is for educational purposes only and should not be considered as professional advice. Always consult with a qualified chemist or scientist for specific guidance.
This post topic: Element