Discover The Element With The Largest Atomic Radius: Which Of These Elements Would Reign Supreme?
Which of These Elements Would Have the Largest Atomic Radius?
Greetings, Element Enthusiast!
Welcome to our informative article where we will explore the intriguing question of which element would have the largest atomic radius. The atomic radius refers to the size of an atom, and understanding this concept is crucial in the world of chemistry and physics. In this article, we will delve into the factors that determine atomic radius and analyze how different elements compare in terms of size. So, let’s embark on this fascinating journey and uncover the answer to our question!
3 Picture Gallery: Discover The Element With The Largest Atomic Radius: Which Of These Elements Would Reign Supreme?
Introduction
1. Understanding Atomic Radius 🌐
Before we dive into which element has the largest atomic radius, let’s first grasp the concept of atomic radius itself. Atomic radius refers to the size of an atom, specifically the distance between the nucleus and the outermost electron shell. It is essential to note that atomic radius can vary due to factors such as electron configuration, atomic number, and the element’s position in the periodic table.
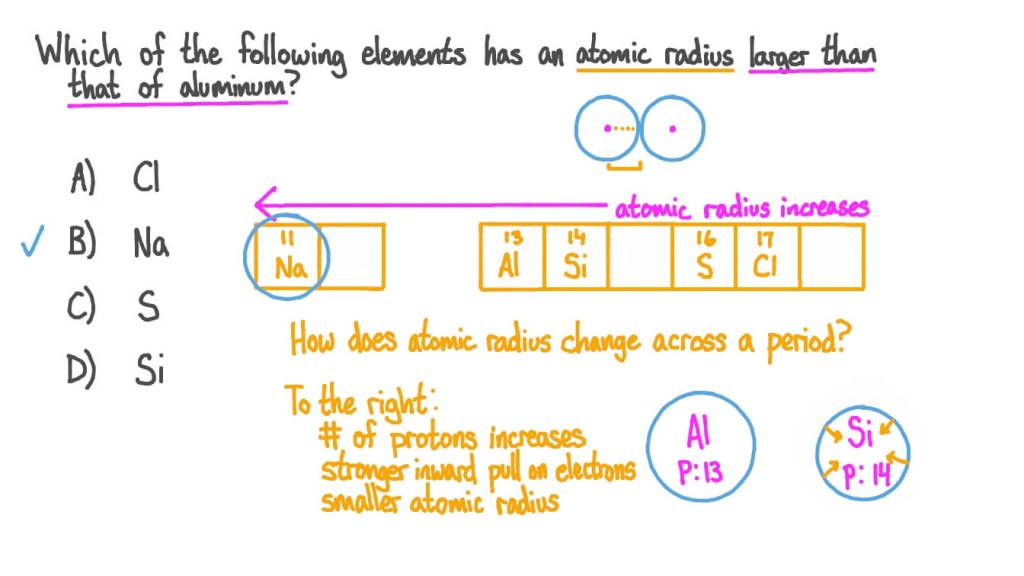
Image Source: nagwa.com
2. Factors Affecting Atomic Radius ⚛️
Atomic radius is influenced by a variety of factors, including electron shielding, effective nuclear charge, electron-electron repulsion, and periodic trends. Electron shielding refers to the repulsion between electrons in different energy levels, which leads to an increase in atomic size. Effective nuclear charge, on the other hand, refers to the positive charge experienced by an electron and affects the attraction between the nucleus and the electrons, thereby influencing atomic radius.
3. Periodic Trend and Atomic Radius 🔄
When examining the periodic table, we observe a general trend in atomic radius. As we move from left to right across a period, the atomic radius decreases due to an increase in effective nuclear charge. Conversely, as we move down a group, the atomic radius generally increases. This trend is primarily attributed to the addition of energy levels or electron shells as we move down the periodic table.

Image Source: nagwa.com
4. The Largest Atomic Radius 📏
Now that we have a solid foundation on atomic radius, let’s explore which element would have the largest atomic radius. Keep in mind that there is no definitive answer, as atomic radius can vary depending on the specific element and its characteristics. However, in general, elements located towards the bottom left of the periodic table tend to have larger atomic radii compared to those towards the top right.
5. Examples of Elements with Large Atomic Radii 🔍
Some examples of elements with relatively large atomic radii include cesium (Cs), francium (Fr), and barium (Ba). These elements are located towards the bottom left of the periodic table and possess additional energy levels, resulting in larger atomic sizes. However, it is important to note that other factors, such as the element’s electron configuration, can also influence atomic radius.
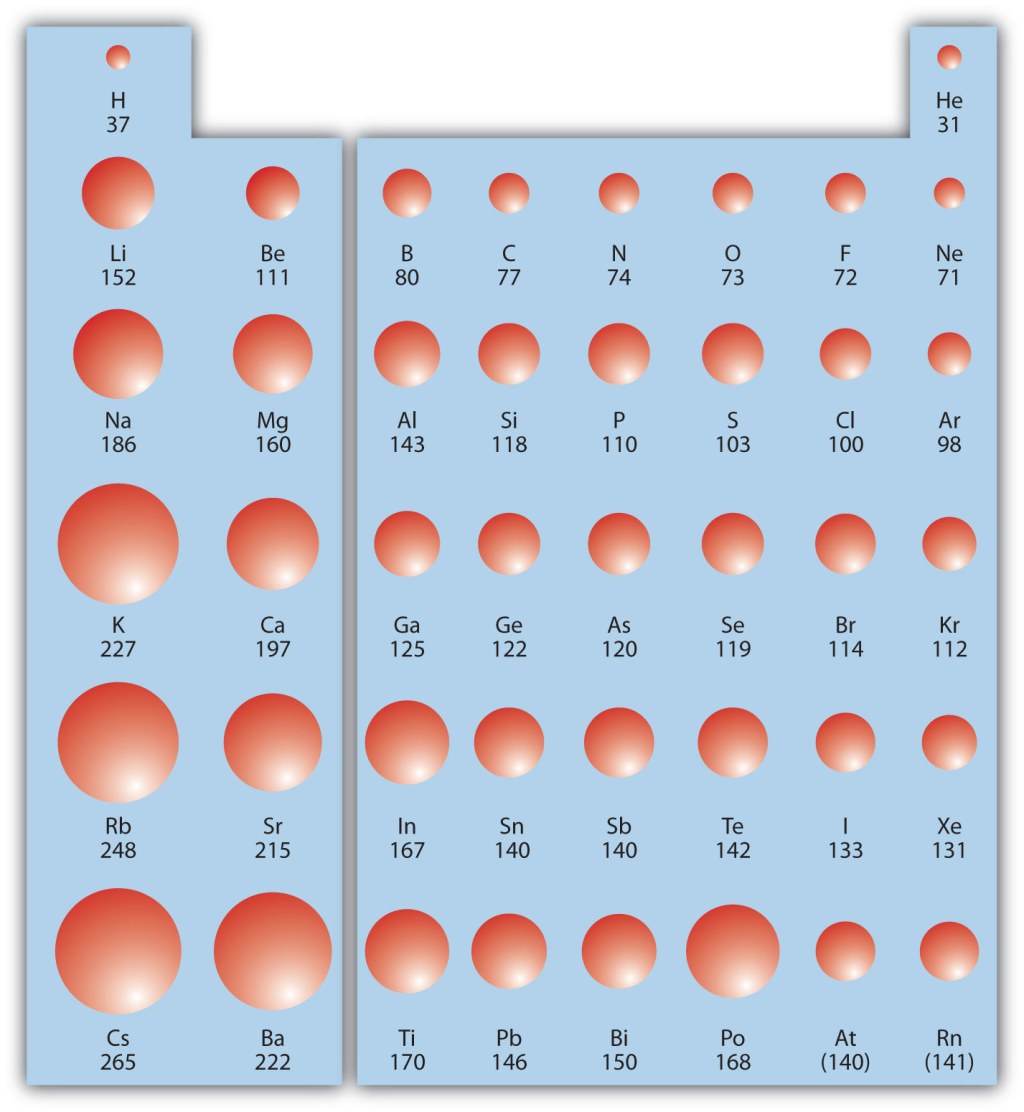
Image Source: socratic.org
6. Experimental Techniques 🧪
Scientists utilize various experimental techniques to measure atomic radius accurately. These techniques include X-ray crystallography, electron diffraction, and the use of atomic force microscopy. Through these methods, researchers can obtain precise data on atomic size, contributing to our understanding of atomic properties.
What Is Atomic Radius?
Atomic radius refers to the size of an atom, specifically the distance between the nucleus and the outermost electron shell. It serves as a fundamental concept in chemistry and physics, influencing the behavior and properties of elements. Atomic radius can vary depending on factors such as electron shielding, effective nuclear charge, and periodic trends.
Electron Shielding
Electron shielding occurs when inner electrons repel outer electrons, reducing the effective nuclear charge experienced by the outer electrons. This repulsion increases the atomic radius by pushing the electron shells farther apart. As a result, elements with more electron shells tend to have larger atomic radii.
Effective Nuclear Charge
Effective nuclear charge refers to the net positive charge experienced by an electron. It is determined by the number of protons in the nucleus and the shielding effect of inner electrons. A higher effective nuclear charge attracts electrons more strongly, resulting in a smaller atomic radius. Conversely, a lower effective nuclear charge leads to a larger atomic radius.
Periodic Trends
Atomic radius exhibits periodic trends in the periodic table. As we move from left to right across a period, the atomic radius generally decreases due to an increase in effective nuclear charge. This trend can be attributed to the addition of protons in the nucleus, which strengthens the attraction between the nucleus and the electrons. However, as we move down a group, the atomic radius increases due to the addition of energy levels or electron shells.
Exceptions and Anomalies
While periodic trends provide a general guideline, there are exceptions and anomalies in atomic radius. Some elements deviate from the expected trend due to unique electron configurations or other factors. For example, transition metals may have smaller atomic radii compared to elements in the same period due to the presence of d-orbitals.
Experimental Determination
Experimental techniques such as X-ray crystallography, electron diffraction, and atomic force microscopy allow scientists to measure atomic radius accurately. These methods provide valuable data that contribute to our understanding of atomic properties and help verify theoretical predictions.
Who Determines the Atomic Radius?
The determination of atomic radius is a collaborative effort among scientists and researchers in the fields of chemistry, physics, and materials science. These individuals conduct experiments, analyze data, and develop theories to better understand atomic properties.
Chemists
Chemists play a vital role in determining atomic radius through experiments and calculations. They utilize various techniques such as X-ray crystallography and electron diffraction to obtain precise measurements of atomic sizes. Chemists also study the trends and patterns in atomic radius, contributing to our understanding of chemical behavior and reactivity.
Physicists
Physicists contribute to the understanding of atomic radius through theoretical calculations and models. They develop mathematical frameworks based on quantum mechanics to describe atomic properties, including atomic radius. Physicists also collaborate with experimentalists to verify predictions and refine theoretical models.
Materials Scientists
Materials scientists focus on the properties and behavior of materials, including atomic arrangements and sizes. They employ techniques such as atomic force microscopy to study the surface structure of materials at the atomic level. By analyzing atomic dimensions, materials scientists can design and engineer new materials with specific properties.
Collaborative Efforts
The determination of atomic radius requires collaboration among chemists, physicists, and materials scientists. By combining experimental data, theoretical models, and computational simulations, these experts enrich our knowledge of atomic properties and contribute to advancements in various fields.
When Was the Concept of Atomic Radius First Introduced?
The concept of atomic radius was first introduced in the early 20th century as scientists began to explore the structure of atoms. It was during this time that researchers discovered the presence of electrons orbiting the atomic nucleus. The idea of atomic radius emerged as scientists sought to understand the spatial distribution of these electrons.
Early Atomic Models
Early atomic models, such as the Bohr model, proposed that electrons occupied specific energy levels or orbits around the nucleus. These models suggested that the distance between the nucleus and the outermost electron shell could be considered as the atomic radius. However, it is important to note that these models were simplified representations of atomic structure and did not account for the complex quantum mechanical nature of electrons.
Quantum Mechanics and Atomic Radius
The development of quantum mechanics in the early 20th century revolutionized our understanding of atomic properties. Quantum mechanics provided a mathematical framework to describe the behavior of electrons and their distribution around the nucleus. This framework allowed for a more accurate definition and calculation of atomic radius based on electron probability densities.
Continual Refinement
Since its introduction, the concept of atomic radius has undergone continual refinement and improvement. The advancement of experimental techniques, such as X-ray crystallography and electron diffraction, has enabled scientists to obtain more accurate measurements of atomic sizes. Additionally, theoretical advancements in quantum mechanics have provided deeper insights into the factors influencing atomic radius.
Where Can You Find Information on Atomic Radius?
Information on atomic radius can be found in various scientific resources, including textbooks, research papers, and online databases. Here are some reliable sources where you can explore and learn more about atomic radius:
Scientific Journals
Scientific journals publish research articles and reviews on atomic properties, including atomic radius. Journals such as Journal of Physical Chemistry, Physical Review Letters, and Journal of the American Chemical Society feature studies and insights from experts in the field.
Textbooks
Chemistry and physics textbooks provide comprehensive explanations of atomic properties, including atomic radius. Look for textbooks written by renowned authors or those recommended by educators to ensure accurate and up-to-date information.
Online Databases
Online databases dedicated to chemistry, physics, and materials science offer a wealth of information on atomic properties. Websites like the National Institute of Standards and Technology (NIST) Chemistry WebBook and the Cambridge Crystallographic Data Centre (CCDC) provide access to extensive databases and tools for exploring atomic radius.
University and Research Institution Websites
Many universities and research institutions maintain websites that provide educational resources on atomic properties. These websites often include lecture notes, presentations, and research publications that cover topics related to atomic radius.
Professional Conferences and Symposia
Professional conferences and symposia in fields such as chemistry and physics feature presentations and discussions on atomic properties. Attending these events allows you to learn from experts, engage in scientific discourse, and stay updated on the latest research in atomic radius.
Why Does Atomic Radius Matter?
Atomic radius plays a crucial role in understanding the behavior and properties of elements. Here are some key reasons why atomic radius matters:
Chemical Reactivity
Atomic radius influences the chemical reactivity of elements. Elements with larger atomic radii tend to be more reactive as their outermost electrons are farther from the nucleus, making them more susceptible to interactions and reactions with other atoms.
Bonding and Molecular Structure
Atomic radius affects bonding and molecular structure. The size of atoms determines the distance between them in a molecule, influencing the strength and type of chemical bonds formed. For example, larger atoms are more likely to form ionic bonds, while smaller atoms are more likely to form covalent bonds.
Physical Properties
Atomic radius is linked to various physical properties of elements. For instance, larger atoms generally have higher melting and boiling points due to stronger interatomic forces. Atomic radius also influences other properties such as density, electrical conductivity, and thermal conductivity.
Periodic Trends
Atomic radius contributes to the periodic trends observed in the periodic table. Understanding these trends allows scientists to predict and explain the behavior and characteristics of elements based on their position in the periodic table.
Materials Science and Engineering
Atomic radius is a crucial parameter in materials science and engineering. Researchers consider atomic size when designing and developing materials with specific properties. By manipulating the atomic radius, scientists can influence properties such as hardness, strength, and conductivity.
How Is Atomic Radius Measured?
Scientists employ various experimental techniques to measure atomic radius accurately. Here are three commonly used methods:
X-ray Crystallography
X-ray crystallography is a technique that involves shining X-rays onto a crystalline sample and analyzing the resulting diffraction pattern. By measuring the angles and intensities of the diffracted X-rays, scientists can determine the arrangement of atoms within the crystal and calculate the average atomic radius.
Electron Diffraction
Electron diffraction utilizes a beam of electrons instead of X-rays to study the structure of materials. The electrons interact with the sample, causing them to scatter and form a diffraction pattern. Analysis of this pattern allows scientists to extract information about the atomic arrangement and calculate atomic radius.
Atomic Force Microscopy
Atomic force microscopy (AFM) is a technique that allows scientists to image surfaces at the atomic level. By scanning a sharp tip across the surface, AFM measures the forces between the tip and the atoms, producing a detailed topographical image. Atomic radius can be estimated by analyzing the tip-sample interactions.
Advantages and Disadvantages of Large Atomic Radius
Advantages
1. Increased Reactivity: Elements with larger atomic radii tend to exhibit increased reactivity, making them more prone to forming chemical bonds and participating in reactions.
2. Enhanced Metallic Properties: Large atomic radius contributes to metallic properties such as malleability, ductility, and electrical conductivity, making these elements valuable in various applications.
3. Greater Shielding Effect: Elements with larger atomic radii provide more electron shielding, reducing the effective nuclear charge experienced by outer electrons and stabilizing the atom.
Disadvantages
1. Decreased Stability: Large atomic radius can lead to decreased stability, particularly in heavier elements. The increased distance between the outermost electrons and the nucleus weakens the attractive forces, making these elements more prone to instability.
This post topic: Element
